Introduction
For this is what the Lord says— he who created the heavens, he is God; he who fashioned and made the earth, he founded it; he did not create it to be empty, but formed it to be inhabited— he says: “I am the Lord, and there is no other.”[1]
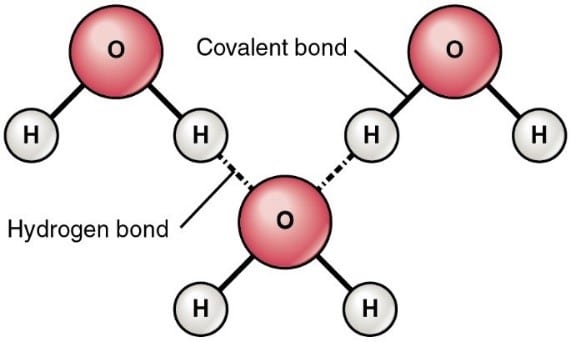
With our Bible verse in mind, there is one form of creation that stands out from everything else and that is water. Its design enables it to have unique properties which are essential for life. These are the result of its structure; two hydrogen atoms joined to one oxygen atom with an angle between them of 104.45 degrees as shown. The molecule is dipolar with the oxygen atom being slightly negative and each hydrogen atom being slightly positive. This separation of charge produces an attractive force between the oxygen atom and a hydrogen atom from another molecule. This attraction is called hydrogen bonding and it brings about water’s unique features.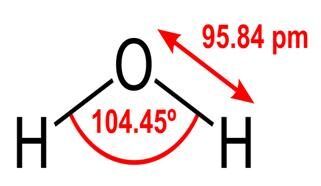
Boiling point and freezing point
Because of hydrogen bonding holding water molecules together, water has extremely high freezing and boiling points when compared with similar dihydrides of elements in the same column of the periodic table where we would expect similar and uniform change of properties. See the table below and notice how both boiling and freezing points decrease as the size of the central atom decreases, but is completely reversed when we come to hydrogen oxide (water).
Compound Boiling Point Freezing Point
Hydrogen Telluride -4ºC -49ºC
Hydrogen Selenide -42ºC -64ºC
Hydrogen Sulphide -62ºC -84ºC
Hydrogen Oxide (water) 100ºC 0ºC
This means that under the temperatures and pressures on the earth’s surface, water is mostly in the liquid form. In fact, water is the only liquid that exists on earth in all of its three forms; solid, liquid and gas. Also, it means that water can evaporate from the oceans at temperatures below its boiling point and fall as rain on the land giving rise to the hydrologic cycle for more on this click here https://www.adefenceofthebible.com/2016/04/16/science-5-the-water-cycle.
Water Expands at 4 ºC
 Water has many unusual properties that are necessary for the existence of life. Like all liquids, water contracts and becomes denser as it cools; but amazingly, this process reverses when water reaches 4˚C. On further cooling, water begins to expand and becomes less dense and lighter, giving rise to the phenomenon that it freezes from the top down, while all other liquids, which continue to become denser as they cool, freeze from the bottom up. It is this property of water that causes bottles to break if
Water has many unusual properties that are necessary for the existence of life. Like all liquids, water contracts and becomes denser as it cools; but amazingly, this process reverses when water reaches 4˚C. On further cooling, water begins to expand and becomes less dense and lighter, giving rise to the phenomenon that it freezes from the top down, while all other liquids, which continue to become denser as they cool, freeze from the bottom up. It is this property of water that causes bottles to break if  they are filled with water and placed in a freezer; it causes icebergs to float, and it is why the sea and even lakes do not freeze completely. This property allows marine life to live. In fact, ice has such good insulating properties that even with the air temperature as low as minus 50˚C, the ice layer can be as little as one meter thick and the water below it will remain liquid. Image credits: Wikimedia commons.
they are filled with water and placed in a freezer; it causes icebergs to float, and it is why the sea and even lakes do not freeze completely. This property allows marine life to live. In fact, ice has such good insulating properties that even with the air temperature as low as minus 50˚C, the ice layer can be as little as one meter thick and the water below it will remain liquid. Image credits: Wikimedia commons.
Transparency
The transparency of water is important for aquatic species, because water is transparent sunlight can pass through it. The energy from the sun is needed by water plants and other water organisms for photosynthesis to live and in doing so, sustain life.
Surface Tension
 Water has other exceptional properties that enable life to exist. One of its wonderful physical properties is surface tension. That is, water sticks to itself very strongly, more strongly in fact than any other liquid except for mercury. And again, this is due to water’s ability to form hydrogen bonds.
Water has other exceptional properties that enable life to exist. One of its wonderful physical properties is surface tension. That is, water sticks to itself very strongly, more strongly in fact than any other liquid except for mercury. And again, this is due to water’s ability to form hydrogen bonds.
This high surface tension can be demonstrated by very carefully placing a needle onto the surface of water contained in a beaker, for example. The needle will float, but as soon as one drop of a surfactant (such as a detergent) is added, the surface tension will be broken and the needle will sink immediately. As well, water’s high surface tension enables small insects to walk on water, rain to break up into small spherical droplets and wood and leaves to float. Water’s high surface tension enables our ecosystem to function. Image credit: https://physics.stackexchange.com/questions/52544/depression-of-water-surface-by-a-needle.
Capillary Action
 When this property is expressed in another form, known as capillary action, it enables plants to transport water from their roots up to their leaves. This applies to all vegetation even very high trees. Capillary action can be demonstrated by placing a flower into colored water and observe the color being transported to the top leaves as shown. Image credit: https://kayjayr-akshay.blogspot.com/2016/09/capillary-action.html?view=flipcard.
When this property is expressed in another form, known as capillary action, it enables plants to transport water from their roots up to their leaves. This applies to all vegetation even very high trees. Capillary action can be demonstrated by placing a flower into colored water and observe the color being transported to the top leaves as shown. Image credit: https://kayjayr-akshay.blogspot.com/2016/09/capillary-action.html?view=flipcard.
Solvent Capacity
As the water is transported, it carries with it, nutrients and this highlights another unique feature of water; its great solvent capacity; greater than any other liquid. Because of its polarity and ability to form hydrogen bonds, water makes an excellent solvent, meaning that it can dissolve many different kinds of molecules. Most of the chemical reactions important to life take place in an aqueous environment inside of cells, and water’s capacity to dissolve a wide variety of molecules is key in allowing these chemical reactions to take place and life to exist.
It is water’s solvent strength that carries nutrients from the roots of vegetation to every part of the plant even to the top of very high trees. Blood is an aqueous system and again, it is water’s solvating power that enables it to transport nutrients throughout the body. Water’s power to be able to dissolve a great range of substances makes life possible.
Viscosity
 Another distinctive property of water that enables capillary action to proceed is its very low viscosity and again, this is due to its ability to form hydrogen bonds. The viscosity of liquids commonly thought of as thickness, is its resistance to flow and with water, it is critical to its many functions. The low viscosity of water is important in its role as the solvent for life’s chemical reactions and for blood circulation.
Another distinctive property of water that enables capillary action to proceed is its very low viscosity and again, this is due to its ability to form hydrogen bonds. The viscosity of liquids commonly thought of as thickness, is its resistance to flow and with water, it is critical to its many functions. The low viscosity of water is important in its role as the solvent for life’s chemical reactions and for blood circulation.
These properties combine to allow water to carry nutrients through very small tubes called capillaries. This same process of carrying nutrients to extremities operates in all forms of life. In animals and people, the process keeps our extremities flushed with oxygen by nutrient-carrying blood.
The image shows water’s low viscosity compared with another liquid. Image credit: https://www.thinglink.com/en-us/scene/436194034428411905.
Heat Capacity
 Water has a high heat capacity; it absorbs a lot of heat before it begins to get hot. To be precise, it takes 4,182 Joules of energy to raise the temperature of one kilogram of water by one degree Celsius. Water’s high heat capacity plays a major role for water in making the earth habitable by absorbing and retaining heat thereby stabilizing the earth’s climate. Water’s high heat capacity enables the earth’s vast amount of water (70 percent of the earth’s surface is covered by water) to moderate its temperature. By contrast, the moon has no water and as a consequence it has an average maximum daytime temperature of 1070C and an average minimum night temperature of minus1800C; conditions under which life could not exist.
Water has a high heat capacity; it absorbs a lot of heat before it begins to get hot. To be precise, it takes 4,182 Joules of energy to raise the temperature of one kilogram of water by one degree Celsius. Water’s high heat capacity plays a major role for water in making the earth habitable by absorbing and retaining heat thereby stabilizing the earth’s climate. Water’s high heat capacity enables the earth’s vast amount of water (70 percent of the earth’s surface is covered by water) to moderate its temperature. By contrast, the moon has no water and as a consequence it has an average maximum daytime temperature of 1070C and an average minimum night temperature of minus1800C; conditions under which life could not exist.
Latent Heat of Vaporization
 Not only does water have a great capacity to absorb heat, it also gives off a lot of heat when it evaporates. This is known as the latent heat of vaporization and it enables us to keep our body temperature at 370C when we exercise, because the hotter we get the more we perspire, and the more we perspire, the more water is evaporated from our bodies taking with it a lot of thermal energy which had been converted to kinetic energy as the gaseous water molecules bounce around, and the more we cool.
Not only does water have a great capacity to absorb heat, it also gives off a lot of heat when it evaporates. This is known as the latent heat of vaporization and it enables us to keep our body temperature at 370C when we exercise, because the hotter we get the more we perspire, and the more we perspire, the more water is evaporated from our bodies taking with it a lot of thermal energy which had been converted to kinetic energy as the gaseous water molecules bounce around, and the more we cool.
Image credit: youthopia.
Summary
The structure of the water molecule that gives it all the properties which facilitate life and provide for a habitable planet did not, and could not, have come about by the accidental random collision of atoms. Oxygen with its six electrons in its outer shell form strong covalent bonds with two atoms of hydrogen using each atom’s single electron to form a molecule with a charge separation and it is this, that creates hydrogen bonding. It is clearly a product of design by the great and benevolent Creator. To deny it is to reject science and to enter the world of make believe.
There is no other liquid like water with respect to its necessity for life—and our earth has it in abundance.
[1] Isaiah 45:18.