Introduction
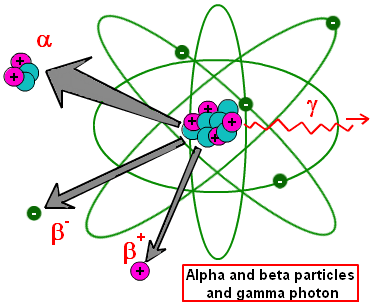 Isotopes are atoms of the same element which vary by the number of neutrons in their nucleus. Some isotopes have so many neutrons that the nucleus is unstable and reduces its size by releasing either alpha or beta particles or gamma rays. In such situations, the isotope is termed radioactive.
Isotopes are atoms of the same element which vary by the number of neutrons in their nucleus. Some isotopes have so many neutrons that the nucleus is unstable and reduces its size by releasing either alpha or beta particles or gamma rays. In such situations, the isotope is termed radioactive.
Radioactive elements are ubiquitous in soil, water and air. Each radioactive element has its own rate of decay, which has been established in laboratories.
The key to radiometric dating is that radioactive isotopes normally decay at a precise rate and the time they take for half of the atoms to decay is known as the half-life. Half-lives can vary from fractions of a second to millions of years for different isotopes. The element that decays is called the parent and the element into which it breaks down is called the daughter. Radiometric dating does not measure dates but rather, it measures the ratio of parent to daughter elements. Then, knowing the rate of decay, it is possible to infer the time the process has taken. However, there are three major and unprovable assumptions involved with this method:
- The initial amount of the daughter element is assumed to be zero.
- The rate of decay is assumed to have been constant for the period of time calculated, which in most cases is in the order of thousands; although evolutionists would claim millions of years.
- It is assumed that the system has remained closed. That is, no parent or daughter elements have entered or left the system during the calculated period.
Because of these assumptions involved in radiometric dating, it is inherently unreliable.
Some examples of radiometric dating
There are many examples, some of which are listed below, where radiometric dating has given the wrong dates for specimens of known historical age.[1]
- Rock from the lava dome at Mt St Helens, which was formed in 1986, was dated by the potassium-argon method as being 350 ± 50 thousand years old.[2]
- Rock from lava flows from Mt Ngauruhoe in New Zealand, which occurred in 1945, 1954 and 1975, was dated from <0.27 to 3.5 million years.[3]
- Wood buried in a basaltic lava flow was variously dated by carbon-14to be 45,000 years old and by K-Ar to be 45 million years old.[4]
- Hawaiian lava flows from eruptions in 1800 and 1801 gave the following dates by the potassium/argon method: 1.41 and 1.60 million years[5]and up to 3 billion years.[6]
- Carbon-14 has a half-life of 5,730 years and consequently, after 57,300 years, that is, 10 half-lives, if no more C-14 is added to the system which is the case with dead plants and animals there would be virtually no C-14 remaining. Radiometric dating laboratories require a sample containing no C-14, for use as a blank. Such a sample cannot be found. Even in coal or diamonds, which are meant to be billions of years old, there are measurable amounts of C-14.[7]
- Fossil wood found in Upper Permian rock that is supposedly 250 million years old still contained C-14.[8]
- A dinosaur bone, supposedly 140 million years old, was sent to the University of Arizona for dating by the carbon-14 The tests were carried out on two samples and gave dates of 9,890 years and 16,120 years.[9]
- Shells from living molluscs were carbon-14dated to 23,000 years.[10]
- A freshly killed seal was carbon-14dated to 1,300 years.[11]
- Shells from living snails were carbon-14dated to 27,000 years.[12]
William Stanfield, Professor of Biological Sciences at California Polytechnic State University, an evolutionist and anti-creationist, concedes in his book:
It is obvious that radiometric techniques may not be the absolute dating methods they are claimed to be. Age estimates on a given geological stratum by different radiometric methods are quite often different, sometimes by hundreds of millions of years. There is no absolutely reliable long-term radiological clock.[13]
Conclusion
 The age of any fossil can never be measured directly, only indirectly with large assumptions being made and almost entirely dependent on the discover’s world view. The cartoon to the right, compliments of Answers in Genesis, makes the point rather sarcastically.
The age of any fossil can never be measured directly, only indirectly with large assumptions being made and almost entirely dependent on the discover’s world view. The cartoon to the right, compliments of Answers in Genesis, makes the point rather sarcastically.
Evolution requires eons of time, hence the inundation of long ages in everything we see and read; millions and even billions of years. Although time makes no difference as far as evolution is concerned because it is based on mutations which only degrade genes, and evolution requires an enormous increase of genetic information in going from a single cell organism (600,000 nucleotides) to human beings (>3 billion nucleotides).
As shown, radiometric dating is of no help to the evolutionist and it even provides strong evidence for a young earth with 14C being in everything. For powerful evidence in support of a young earth, go to: https://www.adefenceofthebible.com/2016/06/05/overwhelming-scientific-evidence-for-a-young-earth.
As shown previously, ice core dating when actual measurements are taken into account, does not support deep time either: https://www.adefenceofthebible.com/2017/05/01/ice-core-dating.
We are told ad nauseam that dinosaurs died out 65 million years ago. This is present in all nature shows, newspaper articles, books, in fact everything we read and see. The 65 Million years is not based on hard science, but merely to prop up evolution. For more on this, go to: https://www.adefenceofthebible.com/2017/02/14/did-dinosaurs-really-become-extinct-65-million-years-ago.
[1] Note: the last three examples involve sea creatures, and their incorrect dates are partly or wholly, due to the ‘Reservoir Effect.’ But this simply explains why the C-14 results are wrong. In fact, all radiometric dates which are wrong have valid explanations, most of which are due to the flawed assumptions behind the method, as explained above. The existence of valid explanations does not change the fact that the method gives wrong answers.
[2] J. Sarfati, Refuting Evolution, Creation Book Distributors, 2002, page 111.
[3] ibid.
[4] A. Snelling, Creation, 1997, 20 (1), pages 24–27.
[5] S. Austin, Creation Ex Nihilo Technical Journal, 1996, 10 (3), 335–343; G. B. Darymple, Earth and Planetary Sciences Letters, 1969, 6, pages 47–55.
[6] J. C. Funkhouser and J. J. Naughton, Journal of Geophysical Research, 1968, 73, pages 4601–4607.
[7] J. Sarfati, Creation, 2006, 28 (4), 26-27). See also page 71.
[8] A. Snelling, Stumping Old Age Dogma, Creation, 1998, 20 (4), pages 48–50; creation.com/stumping-old-age-dogma.
[9] Source: angelfire.com/mi/dinosaurs/carbondating.html, retrieved October 28, 2008.
[10] Science, 1963, 141, pages 634–637.
[11] Antarctic Journal, 1971, 6, page 211.
[12] Science, 1984, 224, pages 58–61.
[13] W. Stanfield, Science of Evolution, MacMillan, 1977, pages 80–84.